Bioelectronic Intelligent Control of Wound Regeneration
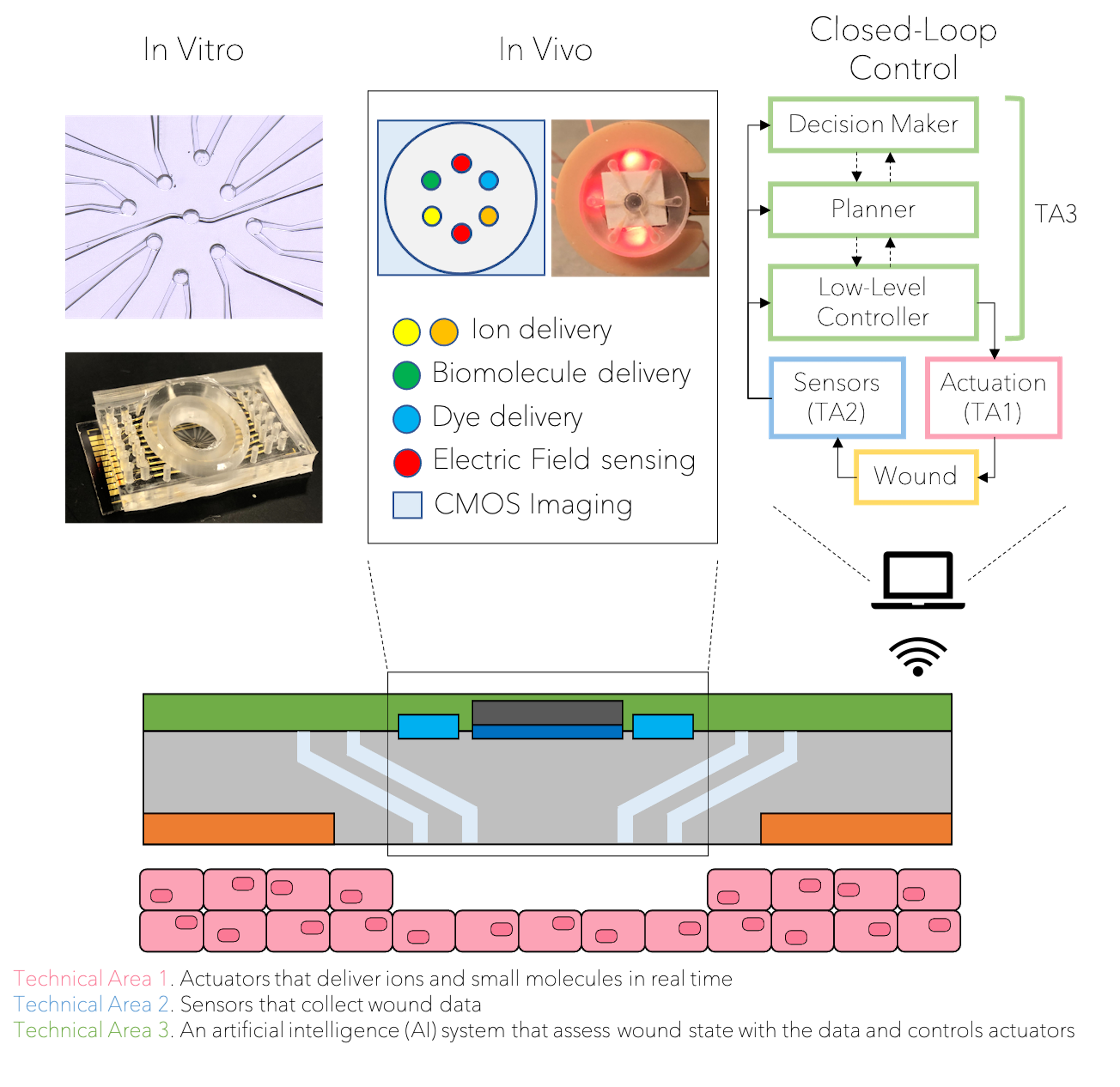 From the simplest unicellular organisms to complex beings, feedback control based on sensing and actuation is a staple of self‐regulation in biological processes and is a key to life itself. Here, we create a closed loop control bioelectronic strategy to expedite the healing of wounds. This strategy is based on sensors that collect wound data, an artificial intelligence (AI) system that assess wound state with the data and creates a personalized on-demand healing recipe, and actuators that deliver ions and small molecules in real time and specific locations to implement the recipe.
From the simplest unicellular organisms to complex beings, feedback control based on sensing and actuation is a staple of self‐regulation in biological processes and is a key to life itself. Here, we create a closed loop control bioelectronic strategy to expedite the healing of wounds. This strategy is based on sensors that collect wound data, an artificial intelligence (AI) system that assess wound state with the data and creates a personalized on-demand healing recipe, and actuators that deliver ions and small molecules in real time and specific locations to implement the recipe.

This research is sponsored by the Defense Advanced Research Projects Agency (DARPA).
Biotronic Control of Vmem and Bioelectric Communication in Bacteria
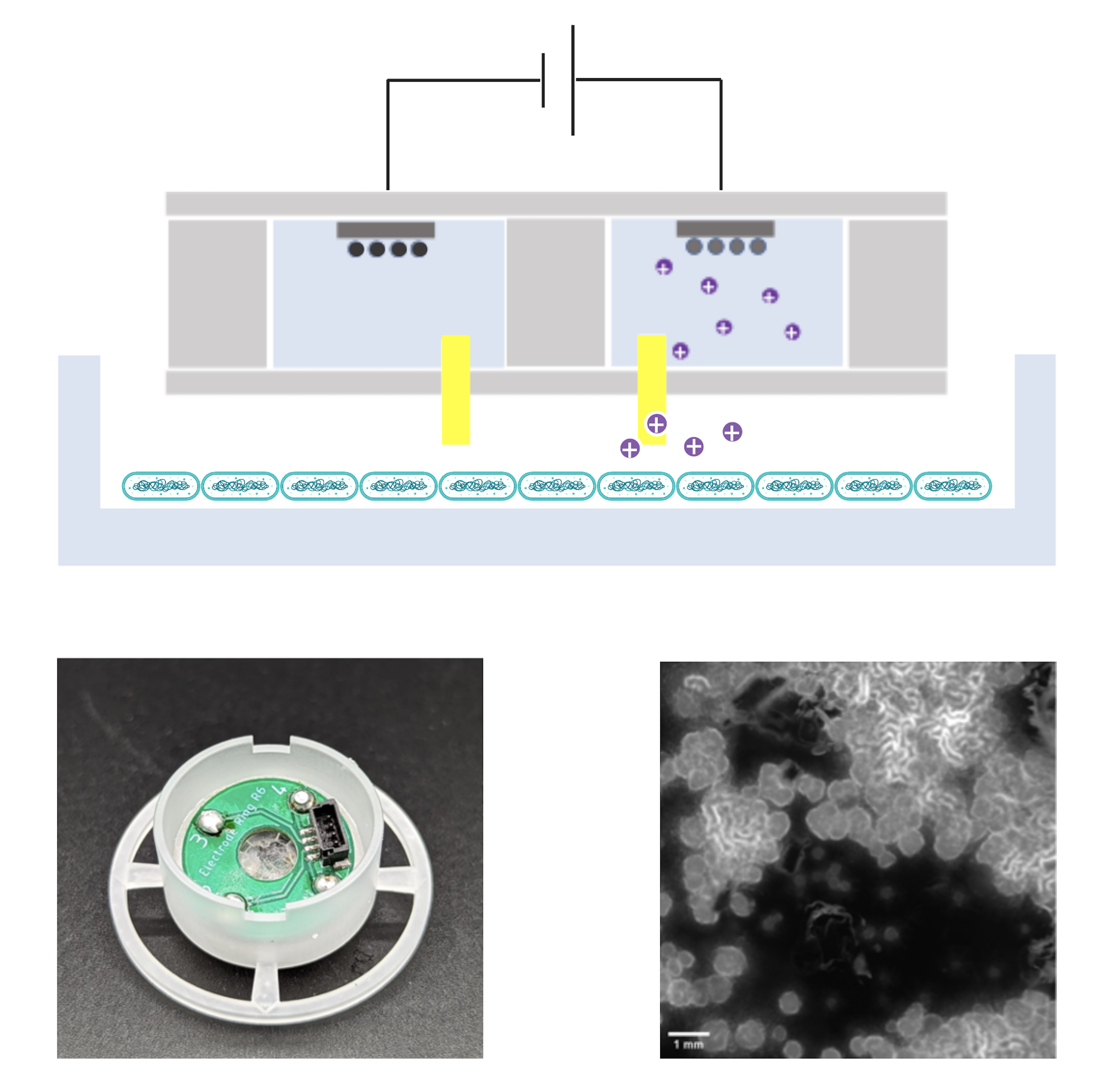 From intercellular communication to organ function, ionic species play an important role in natural systems. A majority of physiological processes involve the exchange of ions between cells. Specific to bacteria, ions play a large role in homeostasis and contribute to the communication among bacteria in a colony. Here, we are developing electrophoretic bioelectronic pumps that accommodate cultures of B. subtilis. These pumps are capable of delivering various ions and nutrients to B. subtilis to study their communication as a function of nutrient continent.
From intercellular communication to organ function, ionic species play an important role in natural systems. A majority of physiological processes involve the exchange of ions between cells. Specific to bacteria, ions play a large role in homeostasis and contribute to the communication among bacteria in a colony. Here, we are developing electrophoretic bioelectronic pumps that accommodate cultures of B. subtilis. These pumps are capable of delivering various ions and nutrients to B. subtilis to study their communication as a function of nutrient continent.

This research is sponsored by the Army Research Office (ARO).

This work was funded by CIRM Shared Stem Cell Facilities (CL1-00506) and CIRM Major Facilities (FA1-00617-1) awards to UCSC.
Previous Research
